Science project
Diffusion, Membranes, and Cryogenics: Can We Freeze Cells?
In this 2-part project on diffusion and cryogenics, I was trying to find out what would pass through membranes. I observed that glucose diffused through a membrane but starch did not. I then used the experience from this investigation to observe what effect salt would have on the preservation of cells. By using salt to allow water to diffuse from a cell, there would be less water in the cell when it expanded upon freezing and thus less damage done to the cell. I thus hypothesized that the greater the salt concentration, the less cell damage done.
I cut a beet into identical weight sections (14 g), and placed then in jars with varying salt concentrations: 0%, 10%, 20%, 30%, 40%, and 50%. I set up 3 sets of these, and placed 1 set in a freezer, 1 set in the refrigerator, and the final set at room temperature. After 24 hours I measured cell damage by measuring the concentration of beet pigment in the water; the rupturing of cells would release the pigment into the water. I used a spectrophotometer to measure the percent transmission of light as a measure of pigment concentration in the water.
There was little cell damage in the refrigerator and none in the room temperature jars, but the freezing caused considerable cell damage. However, when cells were preserved with a salt concentration greater than 20%, there was considerably less damage, and little cell damage at a concentration of 40%.
The significance of these findings is that there is a way to save cells and preserve them. This also indicates that someday, people may be able to freeze animals or humans and bring them back to life.
Problem
What will pass through a cell membrane?
What will pass through a membrane? Starch and sugar are being tested in this experiment to see if they will go through a membrane. The starch molecule is much bigger than the sugar molecule, being that the sugar I used is a simple sugar (glucose). The sugar should be able to go straight through the membrane, however, the starch will be too big to.
I hope to accomplish being able to make a "cell" and really be able to see how a membrane guards the cell. An experiment on cells last year in school prompted my research and sparked my curiosity for chemistry altogether.
Is it possible to preserve cells during freezing?
Being that it is almost impossible to freeze a cell with no protection and still have it live, this project may show a way. Is it possible? It will be (easily) possible to freeze a cell and have it live and function properly.
I hope to accomplish the preservation of cells in freezing conditions. An idea from my science teacher prompted my research. I was very anxious to see how to preserve cells even after freezing them.
Background Information
What Will Pass Through a Membrane?
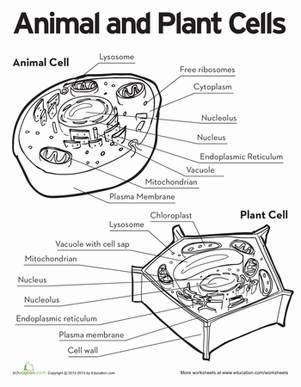
A membrane is a thin layer of lipids (fats) and proteins around a cell. These decide what can come into or come out of a cell. This movement of going into or out of a cell is called osmosis. Naturally particles go from a place of high concentration to a place of low concentration (diffusion). (The particles might not do this if they are forced to move.)
The two groups within carbohydrates are called starches and sugars. The general formula for carbohydrates is (CH2O)n. ("n" is the number of carbons in the backbone) One carbohydrate is called a simple sugar or a monosaccaride. Glucose, like galactose and fructose, is an isomer. An isomer is a compound which differs in structure but not in molecular composition. Disaccharides are two monosaccharides combined in a condensed reaction. Polysaccharides are simple sugar building blocks bonded together to form chains.
Glycogen, or animal starch, is a place to store energy in animals. Glycogen is made up of glucose molecules strung together in a highly branched chain for animals. In plants, however, glucose in the form of a polysaccharide and a monosaccharide is also called a starch. Starches are metabolic reserves which are manufactured by green plants through photosynthesis. (Starches occur in the form of grains.) There are two basic forms of starches, unbranched chains that coil, and branched chains which are similar to glycogen.
Feezing Cells
If the liquid in a cell is not reduced when the cell freezes, then when the water in the cell freezes and expands, the membrane of the cell will break. (A cell membrane is the outermost part of the cell which lets the objects come into or go out of the cell through diffusion.) The inside of the cell will then leak out and the cell dies. (There are some cells in certain animals such as the wood frog which are protected from freezing due to inner body liquids.)
Some water may be taken out of cells that are not protected, through, by using a hypertonic solution. ( Through there are many types of hypertonic solutions; this one would probably contain either sugar or salt in it.) This hypertonic solution would also probably also have to totally surround the cell so that there will be diffusion taking place going out of the cell. Diffusion is the movement going into or out of a cell from a place of high concentration to a place of lower concentration. Not all things, however can diffuse into a cell. The object must be small enough to pass through the cell membrane. (The cell membrane is made of mostly lipids which have very tiny holes in them.)
Hypothesis
What Will Pass Through a Membrane?
I expect to see the glucose go through the membrane and the starch not being able to because a starch molecule is too big. The glucose molecule is a simple sugar and a rather small molecule. It should be able to fit through the membrane easily.
Freezing Cells
I expect to see the salt preserving the beets when they are frozen because the salt will help the water in the beet cell (some of it) to diffuse out. This will leave enough room for the water in the cell to expand and still fit in the cell.
Materials
| What Will Pass Through a Membrane? | Freezing Cells: |
|
*Glucose *Benedict's solution with dropper *Iodine with dropper *Starch *tablespoon *about 30 test tubes *beakers (6 or 7) *tongs *heating surface *goggles *rubber bands and/or tape *membrane *graduated cylinder (10ml.) *test tube rack *labels *camera |
*Beets (1 or 2 small ones) *sharp knife *salt (large container) *18 baby food jars (or at least jars around that size) *spectrophotometer (colorimeter) *a scale that weighs grams *a ruler |
Procedure
| What Will Pass Through a Membrane? | Freezing Cells |
10. Wait overnight and put 5 drops of Benedict's Solution in each test tube that the glucose is in. 11. Place each in a hot water bath and compare how much extra water diffused up with the pictures taken. 12. Put 5 drops of Iodine in each starch beaker, then place them in a hot water bath for 5 minutes. If the color changes darker, then the starch passed through the membrane. |
10. Place a beet cube in each of the jars of 2 of the sets. Leave the third set empty! 11. Place one of the sets with beets in it in the freezer. Place the other two sets in the refrigerator. 12. After a day or two, take all of the beet jars out. 13. Use the spectrophotometer to measure the Absorption and Transmittance percentage for each of the beet jars. Use the light setting 530 nm. Use the set of jars with no beets in them as the blank samples (control). |
Data
Feezing Cells: Effect of Salt Concentration on Cell Damage
See http://www.qacps.k12.md.us/cms/sci/PROJSB.HTM for details.
Discussion
What Will Pass Through a Membrane?
The data in this project tells us where a rough boundary line is of how big a molecule can be and still be able to diffuse through a membrane. This could help us understand why digestion is so important and takes so long to actually happen. This could also tell us what kinds of things you could actually have in your cells.
Freezing Cells
The data for this project tells us how much salt is needed for the preservation of cells after freezing them. Damaged cells release their pigment into the water, thus decreasing light transmission. 0% salt solution after freezing resulted in 0% light transmission, showing almost complete cell damage. In a 20% salt solution, the preservation rate increased dramatically, indicated by an increase in light transmission. At 40% salt content, most cells seem to have been prevented from rupturing. The refrigerator data acts as a control and shows little cell damage, indicted by high light transmission. There was a drop in light transmission at 40% salt and above. I do not have an explanation for this.
The data also tells us that it might be possible to freeze cells and bring them back to life afterwards. This is one of the first steps in the preservation of animals.
Conclusion
What Will Pass Through a Membrane?
My hypothesis that a starch molecule would be too large to go through a membrane was correct. I was also correct that glucose was able to diffuse. This project proves that even though both starches and sugars are carbohydrates, they are different in size. Starch molecules are too complex to diffuse through a membrane. The project proves that not all molecules can pass through a membrane.
This information helps us understand the process of digestion. The food molecules have to be digested to a certain size for the cell to "accept" it.
Freezing Cells
My hypothesis, that the salt would preserve frozen beet cells, was correct. The jars with the most salt in the freezer were only affected by the freezing a little bit, while the jars with little salt were bright purple from dead cells.
This project proves that there is a way to save cells and preserve them. This also proves that one day people might be able to freeze animals and then bring them back to life. The significance of these findings is that if later in the history of the world, people could be frozen, then brought back to life so many years later, they would be able to tell much about history.
My recommendations for future study is to use a variety of cells and try preservation on as many as you possibly can; it may help history! Another recommendation would be to do further tests to see if the frozen cells actually survive after thawing. Also one could use different types of preservation to see what works the best.
Bibliography
http://www.ktca.org/newtons/10/cryogenics.html
Starr, C., Biology: Concepts and Applications, Belmont, CA, 1991, pp. 27 - 29, 40 - 43.
Education.com provides the Science Fair Project Ideas for informational purposes only. Education.com does not make any guarantee or representation regarding the Science Fair Project Ideas and is not responsible or liable for any loss or damage, directly or indirectly, caused by your use of such information. By accessing the Science Fair Project Ideas, you waive and renounce any claims against Education.com that arise thereof. In addition, your access to Education.com's website and Science Fair Project Ideas is covered by Education.com's Privacy Policy and site Terms of Use, which include limitations on Education.com's liability.
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.