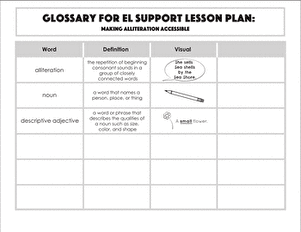
Science project
Making Sugar Crystals
Objective
- Learn how long it takes for sugar crystals to form, based on how saturated the sugar/water solution is.
- Learn how changes in the saturation level affect the rate at which crystals form.
Materials and Equipment / Ingredients
- 2 Cups Of Water
- 3½ Cups Of Sugar
- 1 Cup Measuring Cup
- 4 12 Oz Drinking Glasses
- 4 Pencils Or Coffee Stirrers
- 4 8 Inch Strings
- 4 Paper Clips
- 1 Stirring Spoon
- 1 Tablespoon Measuring Spoon
- Camera (Optional)
- Tea Kettle or Large Microwaveable Bowl
- 4 Pieces Of Masking Tape
- Stove or Microwave to Heat Water
* These materials are all common household supplies.
Introduction
Have you ever seen sugar crystal candy? The candy is made from two simple ingredients: sugar and water. How does the sugar turn from grains of sugar (called granulated sugar) into crystals? How long does it take? Complete this sugar crystal science fair project and learn all about it.
In this experiment, sugar and hot water are stirred together to form a solution. By varying the amount of sugar, the solution may become saturated or supersaturated. As the solution cools, crystals may form.

Photo 1: Granulated Sugar

Photo 2: Sugar Crystals
Research Questions
- What is the right proportion of sugar to water?
- How fast do the sugar crystals form?
- Will crystals form faster when the solution is saturated or supersaturated (see below for definitions)?
Terms, Concepts and Questions to Start Background Research
In this science experiment, sugar and hot water are stirred together to form a solution. By varying the amount of sugar, the solution may become saturated or supersaturated. As the solution cools, crystals may form.
- Solution: The process by which a gas, liquid, or solid is dispersed homogeneously in a gas, liquid, or solid without chemical change. In this case, sugar is our solid which is dissolved uniformly in a liquid, water.
- Saturate: To cause (a substance) to unite with the greatest possible amount of another substance, through solution, chemical combination, or the like. In this case, we create a saturated solution by stirring in the greatest amount of sugar which will dissolve in the water, without leaving visible grains of sugar remaining. The resulting liquid appears to be clear.
- Supersaturate: To increase the concentration of a solution beyond saturation; saturate abnormally. In this case, we can supersaturate our solution by adding more sugar than can be dissolved in the water. The resulting liquid appears cloudy.
- Crystal: a solid body having a characteristic internal structure and enclosed by symmetrically arranged plane surfaces, intersecting at definite and characteristic angles.
Experimental Procedure
- Find out the saturation point of 1 cup of water.
a. Fill a 12 oz-drinking cup with 1 cup of water.
b. Microwave water (or heat it in a kettle) until it starts boiling.
c. Add sugar in one-tablespoon increments until no more dissolves.
d. Record amount of sugar
- Boil two cups of water
- Tie one end of the string to the paper clip, and then the other end of the string to the stirrer.
- Put these on top of the cup
- Label each cup in quarter cup increments down from 1¼ cups of sugar
- Add the designated amount of sugar
- Add ½ a cup of boiling water to each cup
- Stir until dissolved as much as possible
- Immediately after dissolving sugar take pictures
- Record data regularly
- At the end of 2 weeks do final measurements
- Clean up by pouring the sugar water and strings in the trash and cleaning the glasses
Bibliography
Education.com provides the Science Fair Project Ideas for informational purposes only. Education.com does not make any guarantee or representation regarding the Science Fair Project Ideas and is not responsible or liable for any loss or damage, directly or indirectly, caused by your use of such information. By accessing the Science Fair Project Ideas, you waive and renounce any claims against Education.com that arise thereof. In addition, your access to Education.com's website and Science Fair Project Ideas is covered by Education.com's Privacy Policy and site Terms of Use, which include limitations on Education.com's liability.
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.